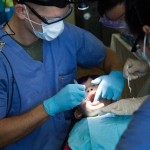
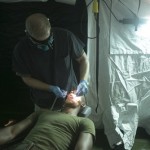
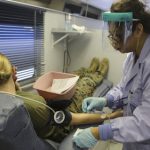
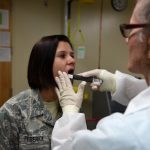
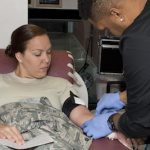
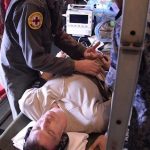
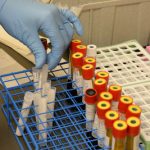
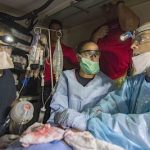
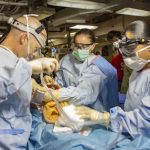
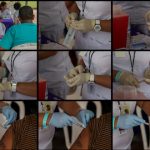
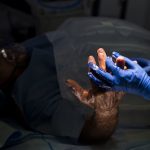
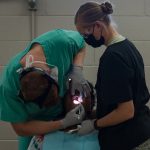
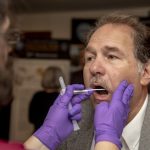
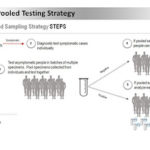
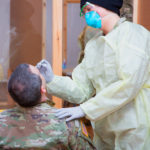
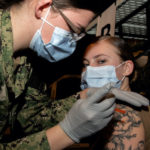
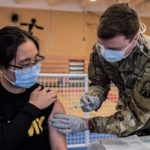
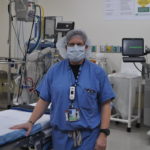
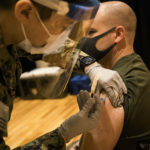
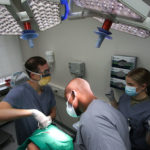
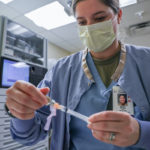
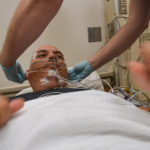
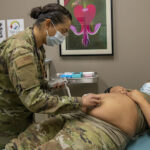
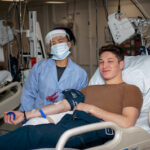
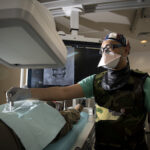
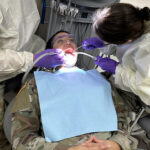
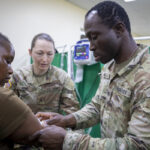
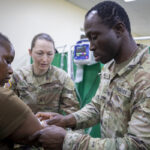
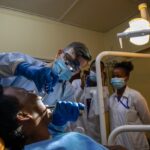
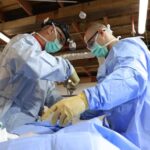
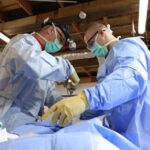
USAMMDA Announces FDA 510 (k) Clearance of Rapid Diagnostic Test for COVID-19
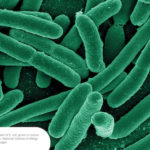
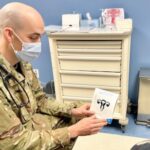
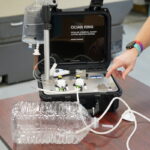
The BioFire COVID-19 Test 2 is an in vitro diagnostic test used in conjunction with the BioFire FilmArray instrument to analyze nasopharyngeal swab specimens from symptomatic individuals suspected of COVID-19 by their healthcare provider. (Photo courtesy of BioFire Defense)