Story by T. T. Parish and Caree Vander Linden
U.S. Army Medical Materiel Development Activity (USAMMDA)
The U.S. Army recently recognized the meritorious performance of the U.S. Army Medical Materiel Development Activity for its contributions to the global fight against the coronavirus.
USAMMDA received the Army Superior Unit Award (ASUA) along with the rest of the U.S. Army Medical Research and Development Command (USAMRDC) team at Fort Detrick, Maryland, according to USAMRDC commanding general, Brig. Gen. Edward Bailey.
“Congratulations to the [USAMRDC team] on being awarded the Army Superior Unit Award for meritorious performance executing the command’s complex mission while supporting the nation’s response to COVID-19,” wrote Bailey in a June 2024 email to staff announcing the achievement. “USAMRDC’s agility and ability to shift resources to support the COVID-19 pandemic response yielded numerous advancements in medical research, testing, screening, and equipment fielding, and helped the nation be well-postured to combat future pandemics and emerging diseases.”
The coronavirus began to spread internationally in late 2019, necessitating a coordinated worldwide response not seen since the Spanish Flu pandemic in the early 1900s. The U.S. response, coordinated and managed by the U.S. Centers for Disease Control and Prevention and aided by government and private health care systems across the country, transformed the national approach to public health crises.
The ASUA recognizes the command’s forward-leaning posture and flexibility in supporting the whole-of-government approach to combating the pandemic, noting USAMRDC’s successful collaborations with DoD, the National Institutes of Health, other government agencies, industry leaders, and laboratories worldwide, according to the award citation issued by the Honorable Christine E. Wormuth, 25th Secretary of the Army.
USAMMDA, which oversees a robust medical development capability to address biological threats and diseases found across the globe, was a vital component of USAMRDC’s all-hands approach to combating the pandemic between March 1, 2020, and Sept. 30, 2021. USAMMDA’s notable contributions to the COVID-19 response included the following:
– Drug treatment: In March 2020, USAMMDA’s Force Health Protection Division entered into a Cooperative Research and Development Agreement with Gilead Sciences to provide the company’s investigational drug, remdesivir, for the treatment of DoD personnel exposed to COVID-19. Under that CRADA, FHP allowed for the investigational use of remdesivir provided by Gilead, at no cost to the government, in the absence of any FDA-approved treatment options. The agreement built on a longstanding partnership between the DoD and Gilead Sciences to develop and test treatments for viral pathogens that pose a risk to military and global health.
– Convalescent plasma: USAMMDA’s Force Health Protection Division closely monitored the initial cases of the novel coronavirus in Wuhan, China, in December 2019. Continued surveillance identified the rapid spread of the disease and the beginning of the associated global pandemic, and FHP pivoted to provide treatment options for Warfighters affected by COVID-19. In May 2020, FHP received approval from the U.S. Food and Drug Administration to implement an Expanded Access Protocol for coronavirus treatment using COVID-19 convalescent plasma (CCP)—blood plasma taken from patients who recovered from COVID-19 and developed protective antibodies. The Navy’s USS Nimitz was the first ship in its fleet to be approved to administer CCP per the treatment protocol, making the effort a notable example of Joint Service collaboration.
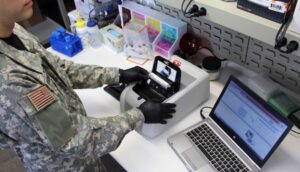
– Coronavirus diagnostics: USAMMDA’s Warfighter Protection and Acute Care Project Management Office partnered with industry developers to manage the BioFire FilmArray® COVID-19 Test 2 cleared for use by the U.S. Food and Drug Administration. The test uses a nasal swab from the patient placed into a tube containing transport medium or saline. Technicians put a small quantity of the fluid into a test pouch and load it into the FilmArray instrument connected to a computer. Test-specific software automates the processing of the sample and delivers results in about 45 minutes. The clinical performance of BioFire COVID-19 Test 2 was evaluated at three clinical study sites in the United States over four months (July through October 2020) during the pandemic.
– Test swabs and respirators: USAMMDA’s Warfighter Expeditionary Medicine and Treatment PMO managed the process for designing and manufacturing 3D-printed nasopharyngeal swabs and N95 respirators to address medical materiel shortfalls across the nation. A standard nasopharyngeal swab, used to safely collect a mucus sample from a patient for laboratory analysis, is integral to diagnostic testing. Because the FDA designates them as medical devices, the 3D-printed swabs had to meet all necessary testing and quality assurance standards in design and manufacturing. N95 respirators are designed to achieve a very close facial fit and efficient filtration of airborne particles. Compared to a surgical mask, which is loose-fitting, the edges of the N95 mask had to form a tight seal around the individual’s nose and mouth to provide the highest level of protection against COVID-19 infection.
– Critical ventilators: USAMMDA’s Warfighter Deployed Medical Systems PMO used its expertise and resources to supply ventilators to treat patients afflicted by COVID-19. As the medical equipping office for the Army, the WDMS team was instrumental in rapidly deploying these much-needed ventilators, as well as infusion pumps, ultrasonic cleaners, intensive care unit sets, blood-gas analyzers, suction apparatuses, steam sterilizers, and other medical supplies with very little lead time.
– Task force support: USAMMDA and USAMRDC program managers supporting the COVID-19 Joint Acquisition Task Force oversaw seven different product groups to focus on replenishing critical national response supplies: ventilators, N95 masks, screening and diagnostics, personal protective equipment, pharmaceuticals, vaccine delivery, and continuous renal replacement therapy. The JATF supported the acquisition mission of the Department of Defense’s COVID-19 response, partnering with the Federal Emergency Management Agency and Department of Health and Human Services to help support their requests for medical resources. The JATF team served as the single entry point to the DoD acquisition enterprise and interagency requests for assistance.
While the end of the pandemic was officially declared in May 2023, the ASUA serves as a capstone celebrating the command-wide efforts to manage the first global health crisis of the 21st century. The lessons of adaptability, safety, commitment to duty, strength in the face of adversity, and collaborative efforts are not lost on USAMMDA’s current commander, Col. Andy Nuce. Nuce joined USAMMDA in June 2022, after U.S. national response efforts led to COVID-19 immunizations and the refocusing of Army medical development initiatives. He is grateful to accept the award on behalf of all who contributed to USAMMDA’s efforts during the height of the pandemic.
“This award signifies the USAMMDA team’s continued focus and unwavering commitment to developing and fielding modern treatment solutions in the face of uncertainty,” said Nuce. “While we continue to focus on equipping U.S. Warfighters across the globe, the pandemic was a stark reminder of why our mission is so important. The team saw the effects of an international crisis every day, the team felt the burden of the unknown, the team experienced the strength found in unity and working toward a common purpose.
“The impact USAMMDA had during the pandemic is still visible today,” he added. “We are more prepared for uncertainty, better established as a joint-force enabler across the spectrum of combat casualty care, and uniquely positioned to respond as a vital contributor to the Army’s medical development, acquisition, and fielding missions.”
Nuce, who is leading USAMMDA’s transition to the Defense Health Agency, cites the pandemic response as a primer for organizational change. The lessons of COVID-19 have helped, in part, to prepare the USAMMDA team for continued excellence despite the variables caused by uncertainty. The ASUA is a fitting recognition of the team’s immediate and lasting contributions to U.S. Army and DoD medical readiness.
“While the pandemic consumed the attention of all who work in health care over the past few years, our mission to continue preparing for the next war or pandemic never ended, and we are better suited to this purpose than ever due to the lessons learned during the COVID-19 response,” said Nuce. “I am incredibly humbled and honored to accept this award on USAMMDA’s behalf, but the credit for our achievements belongs to every member of the team who worked tirelessly while upholding the standards our Army represents. Congratulations to all for this well-deserved recognition.”
About USAMMDA
The U.S. Army Medical Materiel Development Activity, part of the U.S. Army Medical Research and Development Command, develops, delivers, and fields critical drugs, vaccines, biologics, devices, and medical support equipment to protect and preserve the lives of Warfighters across the globe. USAMMDA project managers guide the development of medical products for the U.S. Army Medical Department, other U.S. Services, the Joint Staff, the Defense Health Agency, and the U.S. Special Forces community. The process takes promising technology from DoD, industry, and academia to U.S. Forces, from the testing required for U.S. Food and Drug Administration approval or licensing to fielding and sustainment of the finished product.